Menu
- Home
- Company
- Medical & Dental
- Repellent
- Wound Care
-
Solution
- 【Skin Care】Product Comparison – Sting Free Liquid Bandage vs. Sting Free Barrier Film
- 【Oral Care】A Complete Guide to Mouth Ulcers (Oral Mucositis)
- 【Oral Care】The Benefits of Oral Gel for Cancer Patients with Mouth Ulcers
- 【Mosquito Repellent】What is Zika virus disease?
- 【Teeth Care】How to Prevent Gum (periodontal) Disease?
-
News
- Thank You for Visiting Us at MEDICALL India
- Meet Hannox at PHARMEDI VIETNAM. 24–27 Sep. 2025
- Visit MEDICALL, Chennai Trade Center. 25-27 July 2025
- Hannox sponsored the 32nd "Love Without Borders" Summer Medical Mission to Cambodia
- New product launch - Acnevo Liquid Paste
- The ARAB HEALTH Exhibition, Dubai. 27-30 Jan. 2025
- Hannox sponsored Taipei Medical University to donate supplies to remote districts in eastern Taiwan
- Wecome to be our distributor
- The ARAB HEALTH Exhibition, Dubai. 29 Jan. - 1 Feb. 2024
- Malaria Prevention Solutions for WESTERN AFRICA - DORAS Mosquito Repellent Products
- Spike in dengue cases due to global warming, warns WHO
- MEDICAL FAIR, Germany. 13 - 16 Nov. 2023
- API and drug GDP license
- Doras Mosquito Away 8 Hour Mosquito Repellent Patch
- THANK YOU FOR VISITING US AT MEDICA FAIR, GERMANY 2022
- The ARAB HEALTH Exhibition, Dubai. 30 Jan. - 2 Feb. 2023
- MEDICAL FAIR, Germany. 14 - 17 Nov. 2022
- Looking for global partners.
- New Products Announcement in Nov. 2021
- New Products Announcement in Apr. 2021
- New Products Announcement in Feb. 2021
- Blood glucose monitoring system has been granted approval by TFDA
- New Products Announcement in Nov. 2020
- New Products Announcement in Oct. 2020
- New Products Announcement in Sep. 2020
- New Products Announcement in Aug. 2020
- New Products Announcement in July 2020
- New Products Announcement in Mar. 2020
- New Products Announcement in Feb. 2020
- New Products Announcement in Jan. 2020
- The ARAB HEALTH Exhibition, Dubai. 27 - 30 Jan. 2020
- New Products Announcement in Dec. 2019
- THANK YOU FOR VISITING US AT MEDICA FAIR, GERMANY 2019
- New Products Announcement in Nov. 2019
- New Products Announcement in Oct. 2019
- New Products Announcement in Sep. 2019
- MEDICAL FAIR, Germany. 18 - 21 Nov. 2019
- New Products Announcement in May. 2019
- Thank you for visiting us at The ARAB HEALTH Exhibition, Dubai 2019
- The ARAB HEALTH Exhibition, Dubai. 28 - 31 Jan. 2019
- Thank you for visiting us at Medica Fair, Germany 2018
- MEDICAL FAIR, Germany. 12 - 15 Nov. 2018
- Household Diagnosis Devices
- New Products Announcement in May. 2018
- New Products Announcement in Apr. 2018
- New Products Announcement in Mar. 2018
- New Products Announcement in Feb. 2018
- New Products Announcement in Jan. 2018
- 1/29 - 2/1 2018 The ARAB HEALTH Exhibition, Dubai
- New Products Announcement in Dec.
- How to use D&W Blood Glucose Monitoring System
- How to use DS-Palmlab Blood Glucose Meter
- MoskitoAway 12Hrs Mosquito Repellent Patch
- We're moving!
- How to Use The Infrared Thermometer
- Unlimited Enjoy the Fresh and Pure Air
- How to use Mini TENS Infrared Thermal Knee Brace
- How to use AK-350-IF (Interferential Frequency Current Stimulator)
- How to use Q-PLUS Blood Glucose Meter
- Hannox Advanced Digital Blood Pressure Monitor
- Thank you for visiting us in FIME 2016
- New Products Announcement in Auguest
- 2-4 AUG. 2016 Florida International Medical Expo
- New Products Announcement in July
- Hannox Portable Pain Treatment Stimulator & Beauty Lifter
- New Products Announcement in May
- How to Use the Hannox Nasal Aspirator
- New Products Announcement in Feb.
- 2016 Happy New Year
- 10-12 Sep. 2015 MEDICAL FAIR THAILAND 2015
- 09-11 July 2015 Myanmar Phar-Med Expo 2015
- 16-19 May 2015 The 18th International Exhibition, Iran
- 2013 Indonesia Hospital Expo Post-Show Report
- 2013 Nov. 06-09 Hospital-Expo Jakarta, Indonesia
- 2013 Vietnam Medi-Pharm Expo Post-Show Report
- Vietnam Pharm Expo live
- 2013 Aug. 22-24 Vietnam Medi-Pharm Expo
- Great Finishing at Medica, Dusseldorf 2012.
- Medica Asia 2012 - Singapore
- 2012, Nov 14-17 MEDICA Düsseldorf
- 2012, Sep 12-14 MEDICAL FAIR ASIA SINGAPORE
- Hannox attend IDS DENTAL SHOW FROM March 22 to 26,2011
- Hannox attend MEDICAL FAIR India 2009 , Mar 27~29
- IDS Dental Show 2009
- Prague Dental Days International Congress 2008
- ADA 2008 Dental Exhibition
- Contact Us
Best Sale
Blood glucose monitoring system has been granted approval by TFDA | Medical & Home-Care OEM/ODM Supplier – Private Label - Hannox
Hannox International Corp. is a medical device OEM/ODM and global sourcing partner for blood glucose meters and strips, wound-care dressings, natural mosquito repellents, dental units, and home-care devices. We help hospitals, clinics, distributors, and retailers launch accurate, safe, easy-to-use products.
We manage end-to-end private-label projects—selection, customization, branding and packaging, labeling/IFU, QA/QC, and compliance documentation (ISO/CE where applicable)—through a vetted network of partner factories. Expect clear MOQs, predictable lead times, and consistent quality across diabetes care, wound care, dental, and home-care categories.
Hannox delivers high-quality home-care and patient-monitoring devices, backed by advanced technology and 22 years of expertise. We support OEM/ODM and private-label sourcing—selection, customization, packaging, and compliance—so professional buyers launch standards-ready products fast.
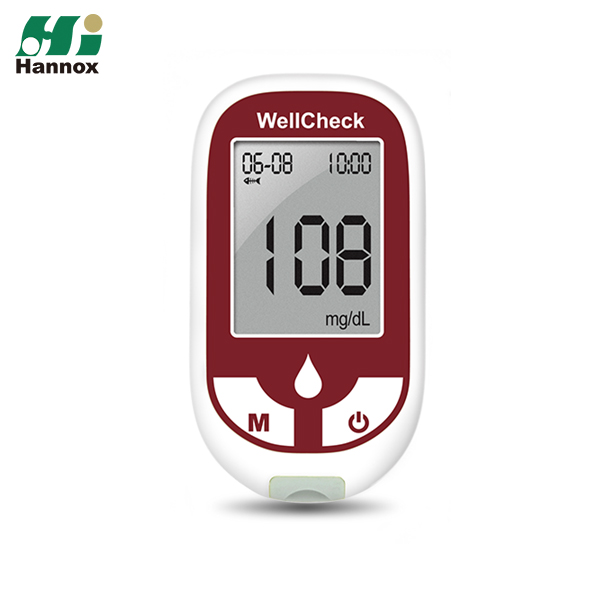
Blood glucose monitoring system has been granted approval by TFDA
2020/12/21 HannoxOur Blood glucose monitoring system (KareU+, KareU, WellCheck) has been granted approval by TFDA.
The quality and accuracy also comply with CE certification.
Please refer to the product description for detailed specifications.
 English
English